How to Verify Them, An Explanation of the Various Certifications
To help everyone better understand the various certification requirements and what each one means, we have assembled the following short guide.
You will encounter the following terms referring to items being certified or approved when dealing with buyers and sellers of PPE and other medical equipment during the COVID-19 pandemic. (NIOSH, FDA Approval, FDA registration, CE mark or CE approved, EUA.)
NIOSH Approval
(NIOSH approval is currently required for N95 Respirators)
NIOSH List – CDC – NIOSH-Approved N95 Particulate Filtering Facepiece Respirators
How to use this list: Search for the manufacturer name first in the first column (for example Shanghai Dasheng Health Products Manufacture Company, Ltd.), then look for the model number in the second column (for example DTC3x). If the model number is on the list it is NIOSH approved (the NIOSH approval number is the third column). If the approval number is bold, then the mask is both NIOSH and FDA approved.
The FDA has recently approved several non-NIOSH options under the Emergency Use Authorizations. See them here – FFRs Manufactured in China.

FDA Approval
“Approval” generally only applies to “high risk” medical devices (Class III), such as ventilators and other machines, parts, and accessories for these, filtering devices (however NIOSH only has been approved for these), PAPR/CAPR, Pharmaceuticals, and test kits, etc. You can search here – Recently-Approved Devices | FDA
Low-risk items are not generally FDA approved, but generally need to be registered. Items in this group include equipment such as goggles, gowns, booties, face shields, and similar items. You can find out by searching for it in this database. – Product Classification
FDA Registration (not approval) – If a device is a class 1 (low risk) device – a premarket notification application and FDA clearance are not required before marketing the device in the U.S. however, these manufacturers are required to register their establishment as well as the item they intend to manufacture. You can check if a company or device is registered here – Establishment Registration & Device Listing
This is where there is a lot of confusion. An FDA approval and FDA registration certificate is not the same thing, so knowing which one is needed where is important! An FDA registration certificate alone for a respirator, ventilator or pharmaceuticals is not ok, but it is for standard class 1 PPE gear. A respirator would be a NIOSH approval number, a ventilator or test kit (class III devices) would require an FDA approval, and basic PPE would require an FDA company/product registration. If a manufacturer is registered as a company, the item type itself must still be registered under their device listings under that company’s name.

Emergency Use Authorizations (EUA’s)
These are the items that have fast-tracked approval for the duration of the crisis. – Emergency Use Authorizations | FDA for a particular category, look for the “appendix” links if you don’t see the device listed in the main category. (You can find currently approved COVID-19 tests here)
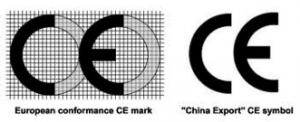
CE Mark of the European Union
The Conformitè Europëenne (CE) Mark is defined as the European Union’s (EU) mandatory conformity marking for regulating the goods sold within the European Economic Area (EEA) since 1985
This mark may well be acceptable for some products, but understanding where the Chinese export economy can be deceptive here is important. In China, where factories pump out substandard imitation goods all too commonly, companies often supply products meant for foreign sale with unsubstantiated “Chinese Export” labels that can look like this:

Here is a comparison of the actual European CE mark with the most common Chinese export mark:
Databases You Can Search
Product classification code search
A list of all medical devices with their associated classifications, product codes, FDA premarket review organizations, and other regulatory information.
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPCD/PCDSimpleSearch.cfm
Establishment registration and device listing
This database includes medical device manufacturers registered with FDA and medical devices listed with the FDA.
Note: Registration of a device establishment, assignment of a registration number, or listing of a medical device does not in any way denote approval of the establishment or its products by FDA.
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm
Listings of CDRH Substantially Equivalent 510(k)s-premarket notifications 2017-2020
https://www.fda.gov/medical-devices/device-approvals-denials-and-clearances/510k-clearances
Recently approved devices 2017-2020
Major medical devices will be found in the approval database (last 4 years is searchable).
De-novo database low-risk items
De novo provides a possible route to classify novel devices of low to moderate risk. This database contains de novo classification orders.
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/denovo.cfm
CDRH Export Certificate Validation (CECV)
This searchable database contains valid export certificates submitted electronically via CECATS (CDRH Export Certification Application and Tracking System) and issued by the Center for Devices and Radiological Health. The results displayed include the facility name, certificate type, expiration date, certificate number, and the number of pages per certificate.
Note: This database is updated once a week.
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/DTLS/rl_cert.cfm
Humanitarian device exemption(HDE)
Humanitarian Device Exemption (HDE) is the FDA process of scientific and regulatory review to evaluate the safety and effectiveness of Class III medical devices. Class III devices are those that support or sustain human life, are of substantial importance in preventing impairment of human health, or which present a potential, unreasonable risk of illness or injury.
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfHDE/hde.cfm
IVD OTC test kits search
A searchable listing of Over-the-Counter tests (OTC) and collection kits that have been cleared or approved by the FDA
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfIVD/Search.cfm